Ferroptosis and Notch Signaling Drive Tumor Progression and Therapeutic Vulnerability
Keywords:
Ferroptosis, Notch, Lipid Peroxidation, Cancer Therapeutic Targets, Metabolic Reprogramming, Immunogenic Cell Death (ICD), Iron metabolism, Precision oncologyAbstract
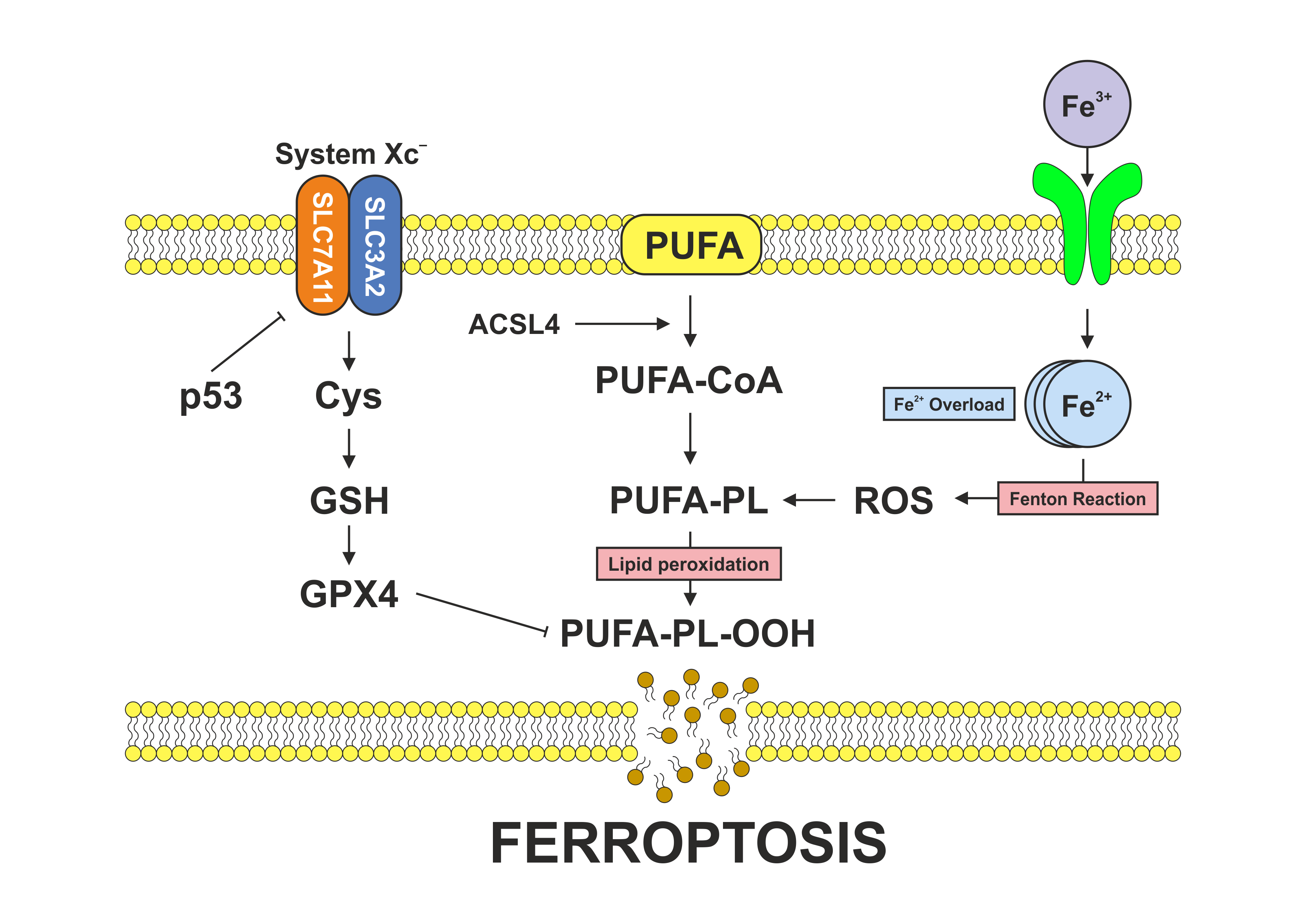
Ferroptosis is an iron-dependent form of regulated cell death characterized by unchecked lipid peroxidation and redox imbalance, which makes cancer cells vulnerable. The Notch signaling pathway is involved in cell survival, proliferation, differentiation, and helps cells adapt to metabolic and oxidative stress. Notch signaling intersects with ferroptosis through specific mechanisms: it modulates iron homeostasis by altering iron transport and storage proteins, influences lipid metabolism by regulating enzymes that modify membrane phospholipids, and affects antioxidant defenses by controlling the expression of genes such as SLC7A11 that regulate glutathione levels. As a result, Notch activity can sensitize cells to ferroptotic death by encouraging iron accumulation and lipid remodeling or confer resistance by increasing antioxidant capacity and reducing oxidative damage. In cancer, alterations in both ferroptosis and Notch signaling contribute to tumor initiation, progression, metastasis, and therapeutic resistance, in part through mechanistic interactions.
Recent studies report a link between ferroptosis and Notch signaling in several tumor types. However, this relationship likely varies by cancer type and experiment. Studying how these pathways connect could reveal new therapeutic targets, particularly in cancers that rely upon Notch-dependent metabolic programs or resist ferroptosis. Future work should address practical concerns. Selecting appropriate cellular targets, refining delivery methods, and understanding the tumor microenvironment will be important before demonstrating clinical benefits. Ultimately, more targeted ways to exploit the ferroptosis-Notch link may expand precision oncology tools. However, this remains under investigation and has not yet been approved as a therapy.
References
1. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. doi:10.1016/j.cell.2012.03.042
2. Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent and AIF-mediated cell death. Cell Metab. 2008;8(3):237–48. doi:10.1016/j.cmet.2008.07.005
3. Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34(4):496–502. doi:10.1016/s0891-5849(02)01360-6
4. Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–8. doi:10.1038/nchembio.2239
5. Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338–43. doi:10.1016/j.bbrc.2016.08.124
6. Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–91. doi:10.1038/ncb3064
7. Metz CW, Bridges CB. Incompatibility of mutant races in Drosophila. Proc Natl Acad Sci U S A. 1917;3(12):673–8. doi:10.1073/pnas.3.12.673
8. Mohr OL. Character changes caused by mutation of an entire region of a chromosome in Drosophila. Genetics. 1919;4(3):275–82. doi:10.1093/genetics/4.3.275
9. Bridges CB. Non-disjunction as proof of the chromosome theory of heredity (concluded). Genetics. 1916;1(2):107–63. doi:10.1093/genetics/1.2.107
10. Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985;43(3 Pt 2):567–81. doi:10.1016/0092-8674(85)90229-6
11. Kidd S, Kelley MR, Young MW. Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol. 1986;6(9):3094–3108. doi:10.1128/mcb.6.9.3094-3108.1986
12. Vässin H, Campos-Ortega JA. Genetic analysis of Delta, a neurogenic gene of Drosophila melanogaster. Genetics. 1987;116(3):433–445. doi:10.1093/genetics/116.3.433
13. Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14(4):295–300. doi:10.1038/nsmb1227
14. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–33. doi:10.1016/j.cell.2009.03.045
15. Wang H, Zou J, Zhao B, Johannsen E, Ashworth T, Wong H, et al. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci U S A. 2011;108(36):14908–13. doi:10.1073/pnas.1109023108
16. Takebe N, Nguyen D, Yang SX. Targeting Notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141(2):140–49. doi:10.1016/j.pharmthera.2013.09.005
17. Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66(10):1631–46. doi:10.1007/s00018-009-8668-7
18. Palmer WH, Deng WM. Ligand-Independent Mechanisms of Notch Activity. Trends Cell Biol. 2015;25(11):697–707. doi:10.1016/j.tcb.2015.07.010
19. Zhao J, Ma W, Wang S, Zhang K, Xiong Q, Li Y, et al. Differentiation of intestinal stem cells toward goblet cells under systemic iron overload stress are associated with inhibition of Notch signaling pathway and ferroptosis. Redox Biol. 2024;72:103160. doi:10.1016/j.redox.2024.103160
20. Ning J, Qiao L. Ferroptosis: molecular mechanisms, pathophysiology, and role in pediatric pulmonary diseases. Cell Biol Toxicol. 2025;41(1):144. doi:10.1007/s10565-025-10100-z
21. Chang C, Wang M, Li J, Qi S, Yu X, Xu J, et al. Targeting NOTCH1-KEAP1 axis retards chronic liver injury and liver cancer progression via regulating stabilization of NRF2. J Exp Clin Cancer Res. 2025;44(1):232. doi:10.1186/s13046-025-03488-3
22. Liu X, Chen C, Han D, Zhou W, Cui Y, Tang X, et al. SLC7A11/GPX4 inactivation-mediated ferroptosis contributes to the pathogenesis of triptolide-induced cardiotoxicity. Oxid Med Cell Longev. 2022;2022:3192607. doi:10.1155/2022/3192607
23. Wei J, Zhu L. The role of glutathione peroxidase 4 in the progression, drug resistance, and targeted therapy of non-small cell lung cancer. Oncol Res. 2025;33(4):863–872. doi:10.32604/or.2024.054201
24. Doll S, Conrad M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life. 2017;69(6):423–434. doi:10.1002/iub.1616
25. Wang Z, Shen N, Wang Z, Yu L, Yang S, Wang Y, et al. TRIM3 facilitates ferroptosis in non-small cell lung cancer through promoting SLC7A11/xCT K11-linked ubiquitination and degradation. Cell Death Differ. 2024;31(1):53–64. doi:10.1038/s41418-023-01239-5
26. Pontel LB, Bueno-Costa A, Morellato AE, Carvalho Santos J, Roué G, Esteller M. Acute lymphoblastic leukemia necessitates GSH-dependent ferroptosis defenses to overcome FSP1-epigenetic silencing. Redox Biol. 2022;55:102408. doi:10.1016/j.redox.2022.102408
27. Shen N, Li M, Fang B, Li X, Jiang F, Zhu T, et al. ALOX15-driven ferroptosis: the key target in dihydrotanshinone I's epigenetic battle in hepatic stellate cells against liver fibrosis. Int Immunopharmacol. 2025;146:113827. doi:10.1016/j.intimp.2024.113827
28. Ye H, Wu L, Liu YM, Zhang JX, Hu HT, Dong ML, et al. Wogonin attenuates septic cardiomyopathy by suppressing ALOX15-mediated ferroptosis. Acta Pharmacol Sin. 2025;46(9):2407-2422. doi:10.1038/s41401-025-01547-1
29. Xie LH, Fefelova N, Pamarthi SH, Gwathmey JK. Molecular mechanisms of ferroptosis and relevance to cardiovascular disease. Cells. 2022;11(17):2726. doi:10.3390/cells11172726
30. Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi:10.1038/s41586-019-1705-2
31. Mishima E, Nakamura T, Zheng J, Zhang W, Mourão ASD, Sennhenn P, et al. DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature. 2023;619(7968):E9–E18. doi:10.1038/s41586-023-06269-0
32. Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–168. doi:10.1016/j.freeradbiomed.2018.05.074
33. Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi:10.1038/nature14344
34. Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD, et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22(3):569–575. doi:10.1016/j.celrep.2017.12.077
35. Pei Y, Qian Y, Wang H, Tan L. Epigenetic regulation of ferroptosis-associated genes and its implication in cancer therapy. Front Oncol. 2022;12:771870. doi:10.3389/fonc.2022.771870
36. Zhang L, Chen F, Dong J, Wang R, Bi G, Xu D, et al. HDAC3 aberration-incurred GPX4 suppression drives renal ferroptosis and AKI-CKD progression. Redox Biol. 2023;68:102939. doi:10.1016/j.redox.2023.102939
37. Wu J, Zhu S, Wang P, Wang J, Huang J, Wang T, et al. Regulators of epigenetic change in ferroptosis associated cancer (Review). Oncol Rep. 2022;48(6):215. doi:10.3892/or.2022.8430
38. Zhang X, Zhang Y, Wei J, Li X, Jiang A, Shen Y, et al. The Role of SLC7A11 in Tumor Progression and the Regulation Mechanisms Involved in Ferroptosis. Cancer Manag Res. 2025;17:2393–2401. doi:10.2147/CMAR.S551549
39. Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26(11):2329–2343. doi:10.1038/s41418-019-0304-y
40. Yang J, Cao XH, Luan KF, Huang YD. Circular RNA FNDC3B protects oral squamous cell carcinoma cells from ferroptosis and contributes to the malignant progression by regulating miR-520d-5p/SLC7A11 axis. Front Oncol. 2021;11:672724. doi:10.3389/fonc.2021.672724
41. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–396. doi:10.1038/s41568-022-00459-0
42. Schnute B, Shimizu H, Lyga M, Baron M, Klein T. Ubiquitylation is required for the incorporation of the Notch receptor into intraluminal vesicles to prevent prolonged and ligand-independent activation of the pathway. BMC Biol. 2022;20(1):65. doi:10.1186/s12915-022-01245-y
43. Miao X, Yue W, Wang J, Chen J, Qiu L, Paerhati H, et al. Unraveling the role of perivascular macrophages in Alzheimer's disease: insights from the crosstalk between immunometabolism and ferroptosis. Curr Neuropharmacol. 2025. Epub ahead of print. doi:10.2174/011570159X417328250908080404
44. Wang SS, Lv HL, Nie RZ, Liu YP, Hou YJ, Chen C, et al. Notch signaling in cancer: metabolic reprogramming and therapeutic implications. Front Immunol. 2025;16:1656370. doi:10.3389/fimmu.2025.1656370
45. Gan B. ACSL4, PUFA, and ferroptosis: new arsenal in anti-tumor immunity. Signal Transduct Target Ther. 2022;7(1):128. doi:10.1038/s41392-022-01004-z
46. Fan H, Paiboonrungruan C, Zhang X, Prigge JR, Schmidt EE, Sun Z, et al. Nrf2 regulates cellular behaviors and Notch signaling in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. 2017;493(1):833–839. doi:10.1016/j.bbrc.2017.08.049
47. Liu S, Liu J, Wang Y, Deng F, Deng Z. Oxidative stress: signaling pathways, biological functions, and disease. MedComm. 2025;6(7):e70268. doi:10.1002/mco2.70268
48. Liu S, Wu H, Zhang P, Zhou H, Wu D, Jin Y, et al. NELL2 suppresses epithelial-mesenchymal transition and induces ferroptosis via notch signaling pathway in HCC. Sci Rep. 2025;15(1):10193. doi:10.1038/s41598-025-94669-9
49. Li Z, Xiao J, Liu M, Cui J, Lian B, Sun Y, et al. Notch3 regulates ferroptosis via ROS-induced lipid peroxidation in NSCLC cells. FEBS Open Bio. 2022;12(6):1197–1205. doi:10.1002/2211-5463.13393
50. Li X, Li Y, Zhang W, Jiang F, Lin L, Wang Y, et al. The IGF2BP3/Notch/Jag1 pathway: A key regulator of hepatic stellate cell ferroptosis in liver fibrosis. Clin Transl Med. 2024;14(8):e1793. doi:10.1002/ctm2.1793
51. Zhu WW, Liu Y, Yu Z, Wang HQ. SLC7A11-mediated cell death mechanism in cancer: a comparative study of disulfidptosis and ferroptosis. Front Cell Dev Biol. 2025;13:1559423. doi:10.3389/fcell.2025.1559423
52. Auberger P, Favreau C, Savy C, Jacquel A, Robert G. Emerging role of glutathione peroxidase 4 in myeloid cell lineage development and acute myeloid leukemia. Cell Mol Biol Lett. 2024;29(1):98. doi:10.1186/s11658-024-00613-6
53. Mathew M, Sivaprakasam S, Dharmalingam-Nandagopal G, Sennoune SR, Nguyen NT, Jaramillo-Matinez V, et al. Induction of oxidative stress and ferroptosis in triple-negative breast cancer cells by niclosamide via blockade of the function and expression of SLC38A5 and SLC7A11. Antioxidants (Basel). 2024;13(3):291. doi:10.3390/antiox13030291
54. Xu J, Bai X, Dong K, Du Q, Ma P, Zhang Z, et al. GluOC induced SLC7A11 and SLC38A1 to activate redox processes and resist ferroptosis in TNBC. Cancers (Basel). 2025;17(5):739. doi:10.3390/cancers17050739
55. Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. doi:10.1007/s13238-020-00789-5
56. Banu MA, Dovas A, Argenziano MG, Zhao W, Sperring CP, Cuervo Grajal H, et al. A cell state-specific metabolic vulnerability to GPX4-dependent ferroptosis in glioblastoma. EMBO J. 2024;43(20):4492–4521. doi:10.1038/s44318-024-00176-4
57. Yang Z, Zhang T, Zhu X, Zhang X. Ferroptosis-related transcriptional level changes and the role of CIRBP in glioblastoma cells ferroptosis. Biomedicines. 2024;13(1):41. doi:10.3390/biomedicines13010041
58. Gersey Z, Osiason AD, Bloom L, Shah S, Thompson JW, Bregy A, et al. Therapeutic Targeting of the Notch Pathway in Glioblastoma Multiforme. World Neurosurg. 2019;131:252–263.e2. doi:10.1016/j.wneu.2019.07.180
59. Zhang S, Li X, Li X, Zhang Z, Zhu K, Guo J. Development of a ferroptosis-related signature and identification of NOTCH2 as a novel prognostic biomarker in pancreatic cancer. Front Immunol. 2025;16:1659652. doi:10.3389/fimmu.2025.1659652
60. Qiao Y, Su M, Zhao H, Liu H, Wang C, Dai X, et al. Targeting FTO induces colorectal cancer ferroptotic cell death by decreasing SLC7A11/GPX4 expression. J Exp Clin Cancer Res. 2024;43(1):108. doi:10.1186/s13046-024-03032-9
61. Liu L, Qiu Y, Peng Z, Yu Z, Lu H, Xie R, et al. Genistein induces ferroptosis in colorectal cancer cells via FoxO3/SLC7A11/GPX4 signaling pathway. J Cancer. 2024;15(20):6741–6753. doi:10.7150/jca.95775
62. Sadagopan NS, Gomez M, Tripathi S, Billingham LK, DeLay SL, Cady MA, et al. NOTCH3 drives fatty acid oxidation and ferroptosis resistance in aggressive meningiomas. J Neurooncol. 2025;175(3):979–991. doi:10.1007/s11060-025-05208-0
63. Ma Y, Zhang X, Alsaidan OA, Yang X, Sulejmani E, Zha J, et al. Long-chain acyl-CoA synthetase 4-mediated fatty acid metabolism sustains androgen receptor pathway-independent prostate cancer. Mol Cancer Res. 2021;19(1):124–135. doi:10.1158/1541-7786.MCR-20-0379
64. Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol. 2013;10(7):405–413. doi:10.1038/nrurol.2013.110
65. Cui K, Wang K, Huang Z. Ferroptosis and the tumor microenvironment. J Exp Clin Cancer Res. 2024;43(1):315. doi:10.1186/s13046-024-03235-0
66. He H, Yu H, Zhou H, Cui G, Shao M. Natural compounds as modulators of ferroptosis: mechanistic insights and therapeutic prospects in breast cancer. Biomolecules. 2025;15(9):1308. doi:10.3390/biom15091308
67. Garg AD, Romano E, Rufo N, Angostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell Death Differ. 2016;23(6):938–951. doi:10.1038/cdd.2016.5
68. Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. doi:10.1186/s13045-020-00946-7
69. Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510(2):278–283. doi:10.1016/j.bbrc.2019.01.090
70. Tang D, Kepp O, Kroemer G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology. 2020;10(1):1862949. doi:10.1080/2162402X.2020.1862949
71. Demuynck R, Efimova I, Naessens F, Krysko DV. Immunogenic ferroptosis and where to find it? J Immunother Cancer. 2021;9(12):e003430. doi:10.1136/jitc-2021-003430
72. Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi:10.1038/s41586-019-1170-y
73. Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. doi:10.1016/j.immuni.2014.10.017
74. Ioannidis M, Tjepkema J, Uitbeijerse MRP, van den Bogaart G. Immunomodulatory effects of 4-hydroxynonenal. Redox Biol. 2025;85:103719. doi:10.1016/j.redox.2025.103719
75. Li JY, Yao YM, Tian YP. Ferroptosis: a trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front Immunol. 2021;12:701163. doi:10.3389/fimmu.2021.701163
76. Meng Y, Zhou Q, Dian Y, Zeng F, Deng G, Chen X. Ferroptosis: a targetable vulnerability for melanoma treatment. J Invest Dermatol. 2025;145(6):1323–1344. doi:10.1016/j.jid.2024.11.007
77. Deng J, Zhou M, Liao T, Kuang W, Xia H, Yin Z, et al. Targeting cancer cell ferroptosis to reverse immune checkpoint inhibitor therapy resistance. Front Cell Dev Biol. 2022;10:818453. doi:10.3389/fcell.2022.818453
78. Wang X, He J, Ding G, Tang Y, Wang Q. Overcoming resistance to PD-1 and CTLA-4 blockade mechanisms and therapeutic strategies. Front Immunol. 2025;3(16):1688699. doi:10.3389/fimmu.2025.1688699
79. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317–331. doi:10.1016/j.cell.2013.12.010
80. Wu C, Zhao Y, Wang J, Ma L. Unraveling the dual nature of GPx4: From ferroptosis regulation to therapeutic innovation in human pathologies. Med Res Arch. 2025;13(8). doi:10.18103/mra.v13i8.6801
81. Sleire L, Skeie BS, Netland IA, Førde HE, Dodoo E, Selheim F, et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene. 2015;34(49):5951–5959. doi:10.1038/onc.2015.60
82. Sato M, Kusumi R, Hamashima S, Kobayashi S, Sasaki S, Komiyama Y, et al. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin's cytotoxicity in cancer cells. Sci Rep. 2018;8(1):968. doi:10.1038/s41598-018-19213-4
83. Yu L, Huang K, Liao Y, Wang L, Sethi G, Ma Z. Targeting novel regulated cell death: ferroptosis, pyroptosis and necroptosis in anti-PD-1/PD-L1 cancer immunotherapy. Cell Prolif. 2024;57(8):e13644. doi:10.1111/cpr.13644
84. Mokhtarpour K, Razi S, Rezaei N. Ferroptosis as a promising targeted therapy for triple negative breast cancer. Breast Cancer Res Treat. 2024;207(3):497–513. doi:10.1007/s10549-024-07387-7
85. Gudsoorkar P, Wanchoo R, Jhaveri KD. Nirogacestat and hypophosphatemia. Kidney Int Rep. 2023;8(7):1478. doi:10.1016/j.ekir.2023.04.023
86. Pietanza MC, Spira AI, Jotte RM, Gadgeel SM, Mita AC, Hart LL, et al. Final results of phase Ib of tarextumab (TRXT, OMP-59R5, anti-Notch2/3) in combination with etoposide and platinum (EP) in patients (pts) with untreated extensive-stage small-cell lung cancer (ED-SCLC). J Clin Oncol. 2015;33(15_suppl):7508. doi:10.1200/jco.2015.33.15_suppl.7508
87. Casulo C, Ruan J, Dang NH, Gore L, Diefenbach C, Beaven AW, et al. Safety and preliminary efficacy results of a phase I first-in-human study of the novel Notch-1 targeting antibody brontictuzumab (OMP-52M51) administered intravenously to patients with hematologic malignancies. Blood. 2016;128(22):5108. doi:10.1182/blood.V128.22.5108.5108
88. Smith DC, Eisenberg PD, Manikhas G, Chugh R, Gubens MA, Stagg RJ, et al. A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clin Cancer Res. 2014;20(24):6295–6303. doi:10.1158/1078-0432.CCR-14-1373
89. Cao J, Chen X, Chen L, Lu Y, Wu Y, Deng A, et al. DHODH-mediated mitochondrial redox homeostasis: a novel ferroptosis regulator and promising therapeutic target. Redox Biol. 2025;85:103788. doi:10.1016/j.redox.2025.103788
90. Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586–590. doi:10.1038/s41586-021-03539-7
91. Ni Y, Liu L, Jiang F, Wu M, Qin Y. JAG1/Notch pathway inhibition induces ferroptosis and promotes cataractogenesis. Int J Mol Sci. 2025;26(1):307. doi:10.3390/ijms26010307
92. Wang L, Zhu Y, Huang C, Pan Q, Wang J, Li H, et al. Targeting ferroptosis in cancer stem cells: a novel strategy to improve cancer treatment. Genes Dis. 2025;12(6):101678. doi:10.1016/j.gendis.2025.101678
93. Kinowaki Y, Taguchi T, Onishi I, Kirimura S, Kitagawa M, Yamamoto K. Overview of ferroptosis and synthetic lethality strategies. Int J Mol Sci. 2021;22(17):9271. doi:10.3390/ijms22179271
94. Mu Y, Fan Y, He L, Hu N, Xue H, Guan X, et al. Enhanced cancer immunotherapy through synergistic ferroptosis and immune checkpoint blockade using cell membrane-coated nanoparticles. Cancer Nano. 2023;14:83. doi:10.1186/s12645-023-00234-2
95. Dhas N, Kudarha R, Tiwari R, Tiwari G, Garg N, Kumar P, et al. Recent advancements in nanomaterial-mediated ferroptosis-induced cancer therapy: Importance of molecular dynamics and novel strategies. Life Sci. 2024;346:122629. doi:10.1016/j.lfs.2024.122629
96. Bhat KP, Vijay J, Vilas CK, Asundi J, Zou J, Lau T, et al. CRISPR activation screens identify the SWI/SNF ATPases as suppressors of ferroptosis. Cell Rep. 2024;43(6):114345. doi:10.1016/j.celrep.2024.114345
97. Guo Q, Xie M, Wang X, Han C, Gao G, Wang QN, et al. Multi-omic serum analysis reveals ferroptosis pathways and diagnostic molecular signatures associated with Moyamoya diseases. J Neuroinflammation. 2025;22(1):123. doi:10.1186/s12974-025-03446-y
98. Li Z, Chen Y, Hou B, Mi Y, Fu C, Han Z, et al. Machine learning constructs a ferroptosis related signature for predicting prognosis and drug sensitivity in lung cancer. Cell Oncol (Dordr). 2025;48(6):1971-1986. doi:10.1007/s13402-025-01121-1
99. Luo Y, Tang L, Zeng Z, Trang D, Mo D, Yang Y. Ferroptosis and cellular senescence-related genes in cervical cancer: mechanistic insights from multi-omics and clinical sample analysis. Transl Oncol. 2025;60:102487. doi:10.1016/j.tranon.2025.102487
100. Hu J, Song F, Kang W, Xia F, Song Z, Wang Y, et al. Integrative analysis of multi-omics data for discovery of ferroptosis-related gene signature predicting immune activity in neuroblastoma. Front Pharmacol. 2023;14:1162563. doi:10.3389/fphar.2023.1162563
101. Lobry C, Oh P, Mansour MR, Look AT, Aifantis I. Notch signaling: switching an oncogene to a tumor suppressor. Blood. 2014;123(16):2451–2459. doi:10.1182/blood-2013-08-355818
102. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi:10.1038/nrm2009
103. Guo M, Niu Y, Xie M, Liu X, Li X. Notch signaling, hypoxia, and cancer. Front Oncol. 2023;13:1078768. doi:10.3389/fonc.2023.1078768
104. Sarmento LM, Barata JT. Therapeutic potential of Notch inhibition in T-cell acute lymphoblastic leukemia: rationale, caveats and promises. Expert Rev Anticancer Ther. 2011;11(9):1403–1415. doi:10.1586/era.11.73
105. Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi:10.1002/hep.28251
106. Nakamura T, Hipp C, Santos Dias Mourão A, Borggräfe J, Aldrovandi M, Henkelmann B, et al. Phase separation of FSP1 promotes ferroptosis. Nature. 2023;619(7969):371–377. doi:10.1038/s41586-023-06255-6
107. Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9):797. doi:10.1038/s41419-020-02998-6
108. Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16(12):3141–52. doi:10.1158/1078-0432.CCR-09-2823
109. Wang H, Zhang Z, Ruan S, Yan Q, Chen Y, Cui J, et al. Regulation of iron metabolism and ferroptosis in cancer stem cells. Front Oncol. 2023;13:1251561. doi:10.3389/fonc.2023.1251561
110. Lu D, Xia B, Feng T, Qi G, Ma Z. The role of cancer organoids in ferroptosis, pyroptosis, and necroptosis: functions and clinical implications. Biomolecules. 2025;15(5):659. doi:10.3390/biom15050659
111. He Z, Liu Z, Wang Q, Sima X, Zhao W, He C, et al. Single-cell and spatial transcriptome assays reveal heterogeneity in gliomas through stress responses and pathway alterations. Front Immunol. 2024;15:1452172. doi:10.3389/fimmu.2024.1452172
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Joanna Pancewicz, Wieslawa Niklinska, Piotr Michal Chodorowski

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors publishing in Cancer Biome and Targeted Therapy retain full copyright of their work. By submitting a manuscript, authors grant the publisher (GCINC Press) a non-exclusive license to publish, distribute, and archive the article, and to identify itself as the original publisher.
License
All articles are published open access under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0).
https://creativecommons.org/licenses/by/4.0/. This license permits unrestricted use, distribution, reproduction, and adaptation in any medium, including for commercial purposes, provided that:
- Proper attribution is given to the original author(s) and source,
- A link to the license is provided, and
- Any changes made are clearly indicated.
Author Rights
Authors retain the right to:
- Use their article in future works (e.g., books, theses, lectures)
- Share and archive the final published version on institutional repositories or personal websites
- Adapt or translate their work, or authorize others to do so, with proper citation
Reuse by Third Parties
Content is licensed under the Creative Commons Attribution 4.0 International License (CC BY 4.0). Third parties may copy, redistribute, remix, transform, and build upon the material for any purpose, including commercial use, provided that appropriate credit is given to the original author(s).
Archiving and Preservation
All articles are made freely available immediately upon publication, without embargo. Cancer Biome and Targeted Therapy is hosted on the Open Journal Systems (OJS) platform, developed by the Public Knowledge Project (PKP). The journal participates in long-term digital preservation through the PKP Preservation Network (PKP PN) using the LOCKSS system. Authors are encouraged to self-archive in institutional repositories, disciplinary archives, and preprint servers in accordance with the license terms.



